Chapter 1: Brief note on Habitat and species distribution
‘Habitat’, the word goes back to late 18th century Latin habitare meaning ‘to live or dwell’. It is an important consideration while discussing the ecosystem comprising of intricate relationships between organisms. Relationships, rather interactions to be more scientific, are of three types, categorically positive, negative and null or neutral. What matters the most, while discussing any interaction is the biotic performance or guild of a species, the species – a unit. Species are collectively those organisms having a shared genome, i.e., common genetic permutations, which allow them to breed and produce fertile offspring. This definition by eminent evolutionary biologist Earnst Mayr needs a special acknowledgment, as this clarifies all the debates regarding a correct definition of species. Theodosius Dobzhansky, another celebrated geneticist came up with morphological species categorization, which also shares a vital direction in evolutionary biology. Individuals of any species interact and utilize resources to perform their vital activities in a spatial constraint or in a given habitat. This population is described by the resource available and the distribution of their habitats. Individuals of a population shows different spatial arrangements within their habitat, they are (Fig. 1) –
- Clumped distribution
- Uniform distribution
- Random distribution
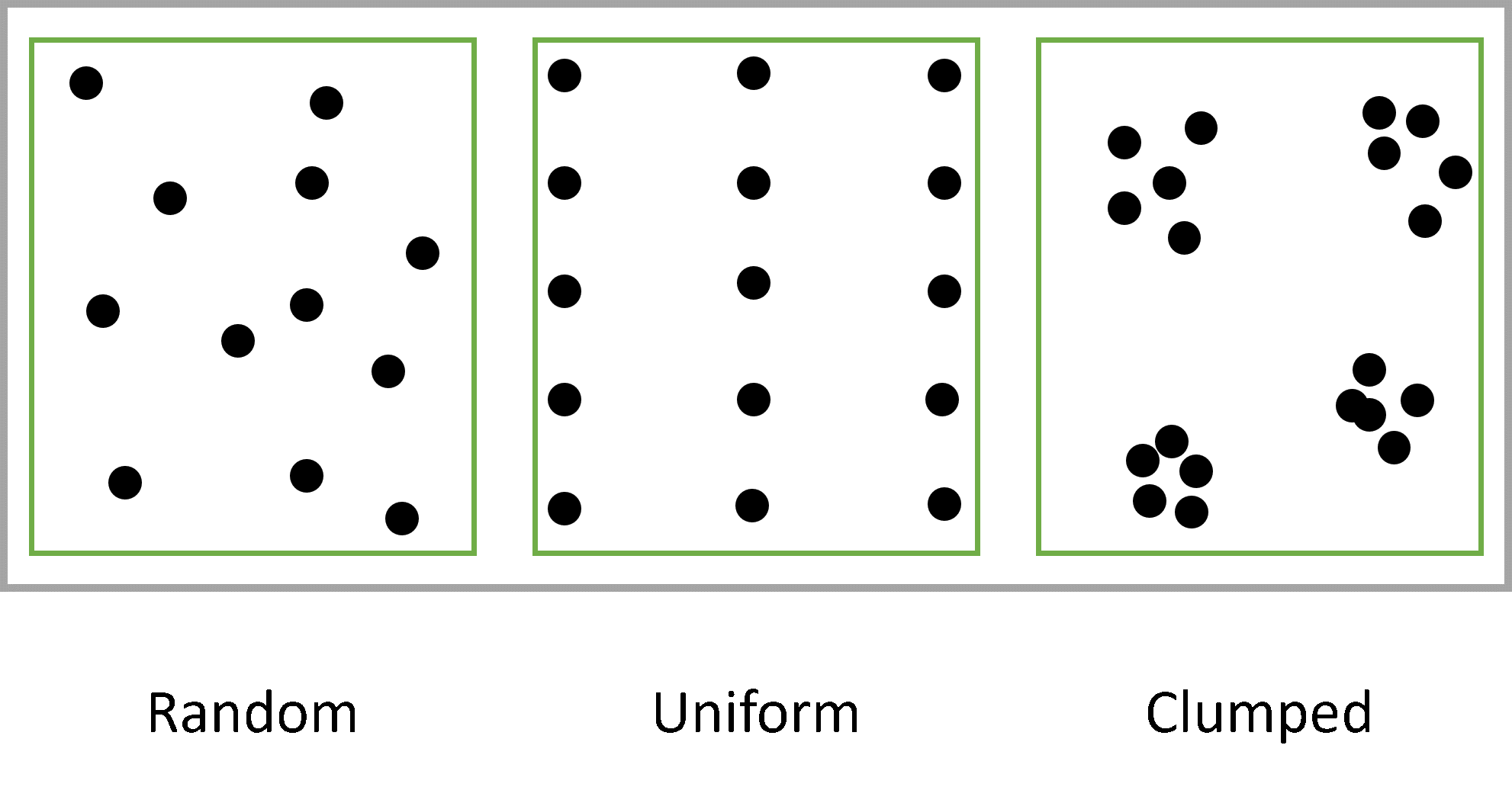
Fig1. Distribution of populations in a habitat
In broader aspect i.e., in landscape concept of ecological distribution we often observe various habitat patches which are either occupied or without any colonization (Wells and Richmond, 1995). These habitat patches in due course of time attain local breeding potential and individuals in that habitat patch start to thrive as spatially discrete groups of subpopulations. In 1970, Richard Levins, ecologist of Harvard University had already coined the term for this subpopulation –metapopulation, population of populations (Fig. 2). This new term describes a collection of local populations that undergoes dispersal, colonization and local extinction. Every population has certain chance of going extinct, so the dynamics of metapopulation depends a lot on the probability of re-colonization.
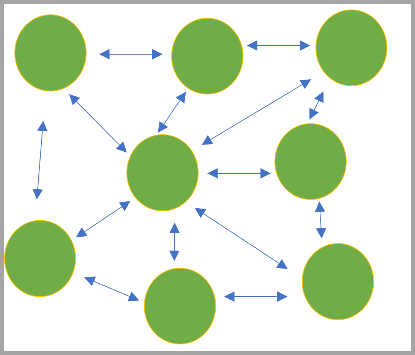
Fig 2. Possible Connectivity between subpopulations
The performance of a habitat begins with two important agenda-
- Habitat selection
- Habitat connectivity (a profoundly important topic for ecological studies nowadays)
Habitat Selection:
Vegetation leads the habitat selection procedure. Starting with the herbivory, vegetation has shaped the relative abundances of animal populations throughout the terrestrial biomes of the world. Plants are critical indicators of suitable habitat patches.
Traditionally, the concept of ‘habitat’ encompassed three fundamental elements: cover, food, and water. Dasmann (1964) went on to categorize cover into two parts: ‘habitat requirements’ and ‘escape cover,’ recognizing that they are not entirely distinct. It was widely acknowledged that vegetation, or cover, plays a crucial role in meeting animals’ essential needs, and altering this vegetation can have a significant impact on wildlife populations.
Wildlife biologists have shown particular interest in understanding the relationships between ‘edge habitats’ and the richness and abundance of wildlife. An edge is defined as the merging of two ‘habitats,’ though it’s worth noting that in this context, earlier researchers were actually referring to the convergence of two vegetation types, commonly known as the edge effect. Aldo Leopold, in his influential work ‘Game Management’ (1933), elevated the edge effect to the status of a natural law, known as his law of interspersion. This principle posits that the boundary between two ‘habitats’ is more favorable as a wildlife habitat than either association on its own. This effect may arise from the merging of distinct environments, each with its own associated wildlife communities.”
Understanding the habitat connectivity:
- Fragmented habitats: In the landscape aspect, we find a habitat fragmented, which often occurs naturally, though in recent trends of anthropogenic activities impose mark on artificial fragmentations too. Habitat loss resulting from this uncontrolled fragmentation leads to immense extermination of biodiversity (Henle et al., 2004). Natural fragments are often found in many tropical forests as forest pockets or patch of forest of distinct or no vegetations different from the preceding or following patch dynamics.
- Natural fragments and manmade fragments: Natural fragmentation shows different rate of resilience to environmental instabilities and can also recover fast. Given the resilience, natural fragmentations differ significantly in respect to the spatial extent and rate of fragmentation from man-made fragmentations; later happens in a fast pace and also causes drastic prolonged isolation of habitats, increase in exotic species(proxy) invasions, predation on existing native biota (Haddad et al., 2015). Among many ecological drawbacks, one notable is the decline in movement of certain species with limited mobility resulting in their attenuation in range and restriction to single patch, causing genetic inbreeding and depression in genetic success likely promoting local extinction (Laurance, 1991).
- Species composition: Floral composition in a habitat patch cannot be considered constant. Ecologically, we define seral changes, seasonal variations and other environmental consequences modifying the species composition temporally (Naeem et al., 1994). In certain studies, we find instances of alien population abundance (as they do not have any natural enemy) causing change in the structure and community framework of certain tropical plant species in reduced fragmented habitat patches (Tabarelli et al., 1999).
In the context of patchy habitats, a very important aspect is dispersal which helps in the long-term balance of the connected populations. Dispersal can be defined as, “Process of individuals leaving the place where they are resident (home) and looking for a new place to live. This behavior can occur both within and between habitat patches” (Robertson, 2020).
Movement between the patches is dynamic and also a selective phenomenon in the wild. The best studied movement is movement via most suited connectivity index of the ecological framework, the Corridors. The route proclaimed as the ‘best suited’ is the route primarily which promotes speedy dispersal and comprises of unselective biota over their range (Perault and Lomolino, 2000). The success of corridor (mostly defined to be linear) or non-linear connectivity index lies in many aspects including its feasibility of genetic connectivity promoting interpatch mating, migration for food, shelter during natural calamities, seed dispersal, wide range animals can freely move and many others (Walker et al., 1997).
Chapter 2: Barren Land Transformation and Habitat Creation
We all know, a forest comprises of various interconnected habitats resided by animals and plants having different levels of susceptibility to extinction. By this, I meant they have different adaptability coefficients. Thus, reforesting or afforesting a barren land should create (re-create) habitats. The level of dependence on such created habitats shall be calculated and determined eventually.
Most important consideration is the plantation of different compositions of native species. Non native exotics have been documented to colonize violtently through mass production of propagules, modify the biota through allelochemical release, alter the habitat and compete for resources. Thus, it is advisable to plant native tree species. Native species are more adapted and produce pristine habitats allowing ecosystem engineers to re-colonize and multiply.
Primary indicator which denotes habitat colonization is reproduction in any form. In case of mammals, home range demarcation is initially the most important step.
We need to measure the ‘biological response’ of the organism inhabiting a developed forest. A ‘biological response’ can be likened to achieved fitness or a related metric. A reforested ecosystem produces broadest possible range of environmental variation, approximately equivalent to a virgin forest. Similarly, organisms’ tolerance levels to different components of variations can be thus measured. Optimal state of environment denotes the highest level of fitness of the organisms in such a transformed forest ecosystem. Occasionally, the truncation might be more sudden or the curve could display an asymmetry, but the fundamental pattern remains intact.
By transforming an otherwise barren land, general considerations like mitigating climate change, generating new pools of carbon, regulating rate of precipitation, enhancing soil nutrition, groundwater recharge etc., can be obtained. But this article tries to focus on habitat building and connectivity patterns, achieved by the transformation.
Landscape designing:
Redesigning any land, in present scenario, needs landscape-level spatial matrix. The rate of colonization and degree of connectedness are the two most important inputs while designing any landscape. We must use different layouts involving trees, herbs, shrubs to enhance the variabilities of abovementioned inputs.
Some Notable Barren Land Transformation Programs by SankalpTaru Foundation-












Reference:
Dasmann, R. F. (1964). Wildlife Biology. John Willey & Sons. Inc., New York, NY. 231pp.
Haddad, N. M., Brudvig, L. A., Clobert, J., Davies, K. F., Gonzalez, A., Holt, R. D., … & Townshend, J. R. (2015). Habitat fragmentation and its lasting impact on Earth’s ecosystems. Science advances, 1(2), e1500052.
Henle, K., Lindenmayer, D. B., Margules, C. R., Saunders, D. A., & Wissel, C. (2004). Species survival in fragmented landscapes: where are we now? Biodiversity & Conservation, 13(1), 1-8.
Laurance, W. F. (1991). Ecological correlates of extinction proneness in Australian tropical rain forest mammals. Conservation Biology, 5(1), 79-89.
Naeem, S., Thompson, L. J., Lawler, S. P., & Lawton, J. H. RM and Woodfin, 1994. Declining biodiversity can alter the performance of ecosystems. Nature, 368(6473), 734.
Perault, D. R., & Lomolino, M. V. (2000). Corridors and mammal community structure across a fragmented, old‐growth forest landscape. Ecological monographs, 70(3), 401-422.
Robertson, D. J. (2020). Corridor Ecology: Linking Landscapes for Biodiversity Conservation and Climate Adaptation, Jodi A. Hilty, Annika TH Kelley, William Z. Lidicker Jr., and Adina M. Merenlender. Natural Areas Journal, 40 (2), 32.
Tabarelli, M., Mantovani, W., & Peres, C. A. (1999). Effects of habitat fragmentation on plant guild structure in the montane Atlantic Forest of southeastern Brazil. Biological conservation, 91(2-3), 119-127.
Walker, R., & Craighead, L. (1997, July). Analyzing wildlife movement corridors in Montana using GIS. In Proceedings of the 1997 ESRI user conference, Redlands, USA.
Wells, J. V., & Richmond, M. E. (1995). Populations, metapopulations, and species populations: what are they and who should care? Wildlife Society Bulletin, 458-462.

